Hot Chocolate Physics
Temperature is a measure of how quickly the tiny molecules of a substance are moving. The study of how quickly a substance heats or cools falls under a branch of science known as thermal physics.
In this activity, we will use energy science to figure out how to make a cup of hot chocolate that stays nice and warm! We will explore the physics of heating and cooling using two common hot chocolate bases: water and milk!
Learning Objectives/Opportunities
Build basic knowledge about the physics of heat energy
Science process skills: controlling variables, hypothesis, observation, data collection
Gentle Disclaimer: This activity includes hot liquids. Be sure to handle any hot materials carefully, as well as any sharp instruments such as food thermometers. Young scientists must be accompanied by their adult science lab partner for all kitchen science experiments.
Materials:
2 identical mugs
8 oz of cold water
8 oz of milk (dairy, almond, oat)
Food thermometer
Clock or timer
Activity Instructions
Fill one mug with 8 oz of cold water and one mug with 8 oz of milk
Take a pre-heat temperature and record
Place one mug in the microwave and heat for 1 minute
Take a post-heat temperature and record
Figure out the change in temperature and record
Check the temperature changes of these two liquids every two minutes as they cool and record
Examining the Experiment:
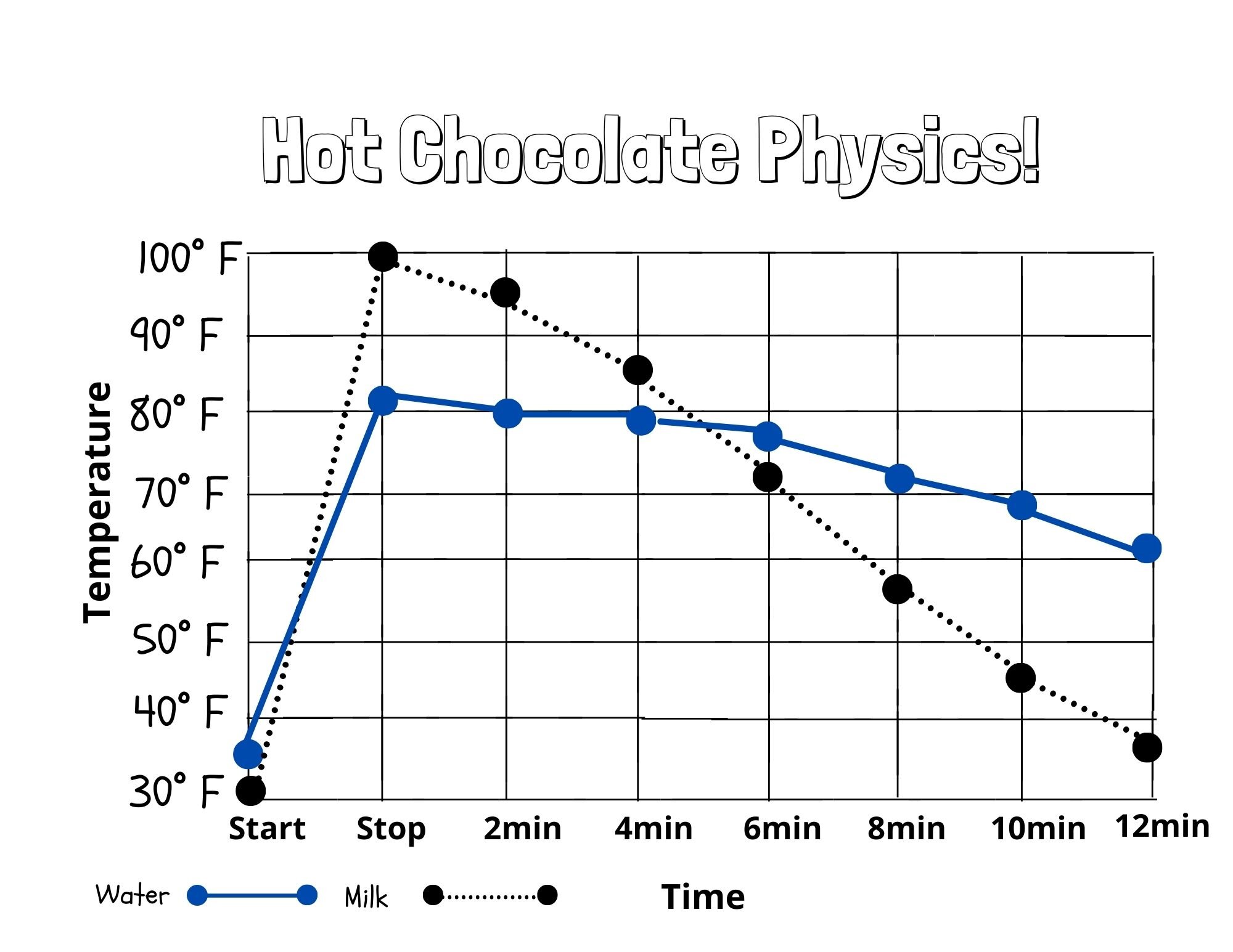
In this activity, we explored the science of heat energy. You should observe that the milk-based hot chocolate heats up quickly and then cooled quickly. Water, on the other hand, heats slower and also cools slower. This is because of the chemical structure of water. Water is one of the only substances on earth that contracts when it heats up and expands when it cools. This gives water its high specific heat point that makes it more resistant to heating up compared to other liquids.
Science Extension: Data Visualization
This activity is a great introduction to data visualization! Use the worksheet below to graph the change in temperature of both liquids to create a simple visual aid.
First, fill in your x-axis (temperature) with the units you are using to measure (°F/C)
Using one color, draw a dot to mark each recorded temperature for milk.
Using a ruler, connect the dots with the same color.
Using a new color, draw a dot to mark each recorded temperature for water
Using a ruler, connect the dots with the same color.
Make a key at the bottom so others will know which line is for milk and which is for water.
STEMSpark Stumper
At the start of this experiment, your liquids were likely starting at different temperatures. How can you get these liquids to the same starting temperature and does that change the results?